Selection of a Suitable Polymeric Matrix for Chronotherapeutic Drug Delivery: A Comparative Study
Keywords:
Chronotherapy, Pulsatile Drug Delivery, Press Coated Tablet.
Abstract
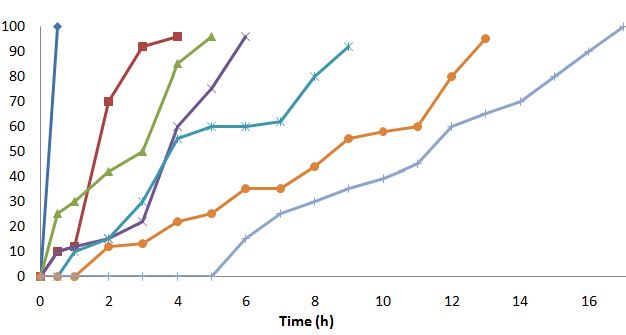
The aim of study was to prepare a chronomodulated press coated tablet having pulse release pattern with the desired lag time for chronic heart disorder. The prepared time-dependent pulse release system consisted of a core drug layer surrounded by polymeric coat. The core consisted of Atenolol (25 mg), while the coating layer consisted of polymer blends of Carbopol 971P:Hydroxypropyl cellulose and Carbopol 971P:Hydroxypropylmethyl cellulose K15M combinations in various ratios. Both combinations were studied for mucoadhasion, swelling. The mucoadhasion for Carbopol 971P:Hydroxypropyl cellulose combination was found to be satisfactory. Increased in the adhesion force was observed as proportion of Carbopol increased for Carbopol 971P:Hydroxypropylmethyl cellulose K15M combination. The swelling rate was increased in both combinations as Carbopol 971 increased. For drug release study pulsatile release pattern was observed for Carbopol 971P:Hydroxypropyl cellulose with lag time up to 12 h. For Carbopol 971P:Hydroxypropylmethyl cellulose K15M combination pulse and sustained release pattern was observed with lag time up to 7 h and sustained release over a period of 16 h. From the obtained results of polymer studies and in vitro release profile, the tablet prepared with Carbopol 971P:Hydroxypropyl cellulose combination was found to be more suitable for the development of a chronotherapeutic drug delivery system.References
1. Lemmer B. Chronopharmacokinetics: Implication for drug treatment. J Pharm Pharmacol 1999;5:887-90.
2. Cheien YW. Novel drug delivery system. 2nd edition. Vol II. New York: Marcel Dekker;1992.
3. Yoshida RS, Okano T, Sakurai Y. Pulsatile drug delivery system using hydrogels. Adv Drug Deliv Rev 1993;11:85-108.
4. Jain NK. Controlled and novel Drug delivery. First edition. CBS Publishers. New Delhi. 1997.
5. Kaifa T, Iisalo E, Selectivity of Acebutolol, Atenolol and Metoprolol in healthy volunteers estimated by the extent the drug occupy β2 receptors in the circulating plasma. J Indian Phrmacol 1993;33:959-66.
6. Jeffer TA, Webster J. Petrie JC. Barker NP. Once daily dosing with Atenolol in patients with mild to moderate hypertension. Brit J Pharmacol 1976;1:990-5.
7. Zang Y, Zang Z, Fangu W. A novel pulse release system based on swelling and osmotic pumping mechanism. J Control Release 2003;89:47-55.
8. Blanchette J, Kavimandan N, Peppas NA. Drug delivery and drug efficiency principle of trance mucosal delivery of the therapeutic agents. Biomedicines and pharmacotherapy. 2004;58:142-151.
9. D’Emanuele A. Responsive polymeric drug delivery. Clin Pharmackinet 1996;31:318-25.
10. Eiji F, Uemura K, Kobatashi M. Studies on applicability of press coated tablet using hydroxypropylcellulose (HPC) in the outer shell for time release preparations. J Control Release 2002;68:215-23.
11. Karavas E, Georgarakis E, Bikiaris D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur J Pharm Biopharm 2006;64:115-26.
12. Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: The next generation. J Pharm Sci 2000;89:850-63.
13. Park H, Robinson JR. In vitro assessment of bioadhesive dosage forms. J Control Release 1985;2:47-56.
14. El- Kamel A, Sokar M, Nagger V, Al Gamal S. Chitosan and sodium alginate based bioadhesive vaginal tablets. AAPS 2002;4:1-8.
15. Kaou HJ, Amodon GL, Lee PI. pH dependent swelling and solute diffusion characteristic of poly (hydroxyehtyl methacrylate- co -methacrylic acid) hydrogels. Pharma Res 1988;5:592-7.
16. Lindberg NO, Berdal A, Enstad G, Seifert E. Investigation of flow properties of powders by means of a uniaxial tester in relation to direct tablet compression. Drug Develop Ind Pharm 2002;28:15-28.
17. Nakhat PD, Babla IB, Khan S, Rathi LG, Ghule BV, Yeole PG. Design and characterization of buccoadhesive tablets of Promethazine hydrochloride. Indian Drugs 2006;44:520-8.
18. Sungthongjeen S, Puttipipatkhachorn S, Paeratakul O, Dashevsky A, Bodmeier R. Development of pulsatile release tablets with swelling and rupturable layers. J Control Release 2004;95:147-59.
19. Duchene D, Touchard F, Peppas NA. Pharmaceutical and medical aspect of bioadhesive systems for drug administration. Drug Develop Ind Pharm 1988;4:283-318.
20. Gandhi RB, Robinson JR. Bioadhesion in drug delivery. Indian J Pharm Sci 1988;50:145-52.
21. Peppas NA, Buri PA. Surface, interfacial and molecular aspect of polymer bioadhesion on soft tissue. J Control Release 1985;2:257-75.
22. Chickering III DE, Mathiowitz E. Bioadhesive drug delivery system. New York, Maecel Dekker. 1999.
23. Madsen F, Eberth K, Smart JD. A rheological assessment of the nature of interaction between mucoadhesive polymers and a homogenized mucus gel. Biomaterials 1998:19:1083-92.
24. Mark H. The use of bioadhesives in targeted delivery within the gastrointestinal tract. Adv. Drug Deliv Rev 1993;11:221-5.
25. Colombo P, Bettini R, Santi P, Ascentiis De A., Peppas NA. Analysis of the swelling and release mechanism from drug delivery systems with emphasis on drug solubility and water transport. J Control Release 1996;39:231-7.
26. Brannon-Peppas Lisa. Peppas NA. Solute and penetrant diffusion in swellable polymers. IX. The mechanism of drug release from pH-sensitive swelling controlled systems. J Control Release 1989;8:267-74.
27. Kaou JH, Amodon GL, Lee PI. pH dependent swelling and solute diffusion characteristic of poly hydroxyehtyl methacrylate-co-methacrylic acid hydrogels. Pharma Res 1988;5:592-7.
28. Devi KP, Rao KVR, Baveja S. Fathi M, Roth M. Zero order release formulation of Oxprenolol hydroxichloride with swelling and erosion control. Pharma Res 1989;6:313-7.
29. Colombo P, Bettini R, Massimo G, Catellani PL, Shanti P, Peppas NA. Drug diffusion front movement is important in drug release control from swellable matrix tablet. J Pharm Sci 1995;84:991-7.
30. Shah SS, Kulkarni MG, Mashelkar RA. pH dependent zero order release from glassy hydrogels: Penetration vs. diffusion control. J Control Release 1991;15:121-32.
31. Nguyen TH, Higuchi T, Himmelstein JK. Erosion characteristic of catalysed poly (ortho esters) matrices. J Control Release 1987;5:1-12.
32. Matsuo M, Nakamura C, Arimori K, Nakano M, Evaluation of hydroxyethylcellulose as a hydrophilic swellable material for delayed release tablets. Chem Pharm Bull 1995;43:311-4.
33. Lin HK, Lin SY, Li MJ, Compression force and amount of outer coating layer affecting the time controlled disintegration of the compression coated tablet prepared by direct compression with micronized ethylcellulose. J Pharm Sci 2001;90:2005-9.
2. Cheien YW. Novel drug delivery system. 2nd edition. Vol II. New York: Marcel Dekker;1992.
3. Yoshida RS, Okano T, Sakurai Y. Pulsatile drug delivery system using hydrogels. Adv Drug Deliv Rev 1993;11:85-108.
4. Jain NK. Controlled and novel Drug delivery. First edition. CBS Publishers. New Delhi. 1997.
5. Kaifa T, Iisalo E, Selectivity of Acebutolol, Atenolol and Metoprolol in healthy volunteers estimated by the extent the drug occupy β2 receptors in the circulating plasma. J Indian Phrmacol 1993;33:959-66.
6. Jeffer TA, Webster J. Petrie JC. Barker NP. Once daily dosing with Atenolol in patients with mild to moderate hypertension. Brit J Pharmacol 1976;1:990-5.
7. Zang Y, Zang Z, Fangu W. A novel pulse release system based on swelling and osmotic pumping mechanism. J Control Release 2003;89:47-55.
8. Blanchette J, Kavimandan N, Peppas NA. Drug delivery and drug efficiency principle of trance mucosal delivery of the therapeutic agents. Biomedicines and pharmacotherapy. 2004;58:142-151.
9. D’Emanuele A. Responsive polymeric drug delivery. Clin Pharmackinet 1996;31:318-25.
10. Eiji F, Uemura K, Kobatashi M. Studies on applicability of press coated tablet using hydroxypropylcellulose (HPC) in the outer shell for time release preparations. J Control Release 2002;68:215-23.
11. Karavas E, Georgarakis E, Bikiaris D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur J Pharm Biopharm 2006;64:115-26.
12. Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: The next generation. J Pharm Sci 2000;89:850-63.
13. Park H, Robinson JR. In vitro assessment of bioadhesive dosage forms. J Control Release 1985;2:47-56.
14. El- Kamel A, Sokar M, Nagger V, Al Gamal S. Chitosan and sodium alginate based bioadhesive vaginal tablets. AAPS 2002;4:1-8.
15. Kaou HJ, Amodon GL, Lee PI. pH dependent swelling and solute diffusion characteristic of poly (hydroxyehtyl methacrylate- co -methacrylic acid) hydrogels. Pharma Res 1988;5:592-7.
16. Lindberg NO, Berdal A, Enstad G, Seifert E. Investigation of flow properties of powders by means of a uniaxial tester in relation to direct tablet compression. Drug Develop Ind Pharm 2002;28:15-28.
17. Nakhat PD, Babla IB, Khan S, Rathi LG, Ghule BV, Yeole PG. Design and characterization of buccoadhesive tablets of Promethazine hydrochloride. Indian Drugs 2006;44:520-8.
18. Sungthongjeen S, Puttipipatkhachorn S, Paeratakul O, Dashevsky A, Bodmeier R. Development of pulsatile release tablets with swelling and rupturable layers. J Control Release 2004;95:147-59.
19. Duchene D, Touchard F, Peppas NA. Pharmaceutical and medical aspect of bioadhesive systems for drug administration. Drug Develop Ind Pharm 1988;4:283-318.
20. Gandhi RB, Robinson JR. Bioadhesion in drug delivery. Indian J Pharm Sci 1988;50:145-52.
21. Peppas NA, Buri PA. Surface, interfacial and molecular aspect of polymer bioadhesion on soft tissue. J Control Release 1985;2:257-75.
22. Chickering III DE, Mathiowitz E. Bioadhesive drug delivery system. New York, Maecel Dekker. 1999.
23. Madsen F, Eberth K, Smart JD. A rheological assessment of the nature of interaction between mucoadhesive polymers and a homogenized mucus gel. Biomaterials 1998:19:1083-92.
24. Mark H. The use of bioadhesives in targeted delivery within the gastrointestinal tract. Adv. Drug Deliv Rev 1993;11:221-5.
25. Colombo P, Bettini R, Santi P, Ascentiis De A., Peppas NA. Analysis of the swelling and release mechanism from drug delivery systems with emphasis on drug solubility and water transport. J Control Release 1996;39:231-7.
26. Brannon-Peppas Lisa. Peppas NA. Solute and penetrant diffusion in swellable polymers. IX. The mechanism of drug release from pH-sensitive swelling controlled systems. J Control Release 1989;8:267-74.
27. Kaou JH, Amodon GL, Lee PI. pH dependent swelling and solute diffusion characteristic of poly hydroxyehtyl methacrylate-co-methacrylic acid hydrogels. Pharma Res 1988;5:592-7.
28. Devi KP, Rao KVR, Baveja S. Fathi M, Roth M. Zero order release formulation of Oxprenolol hydroxichloride with swelling and erosion control. Pharma Res 1989;6:313-7.
29. Colombo P, Bettini R, Massimo G, Catellani PL, Shanti P, Peppas NA. Drug diffusion front movement is important in drug release control from swellable matrix tablet. J Pharm Sci 1995;84:991-7.
30. Shah SS, Kulkarni MG, Mashelkar RA. pH dependent zero order release from glassy hydrogels: Penetration vs. diffusion control. J Control Release 1991;15:121-32.
31. Nguyen TH, Higuchi T, Himmelstein JK. Erosion characteristic of catalysed poly (ortho esters) matrices. J Control Release 1987;5:1-12.
32. Matsuo M, Nakamura C, Arimori K, Nakano M, Evaluation of hydroxyethylcellulose as a hydrophilic swellable material for delayed release tablets. Chem Pharm Bull 1995;43:311-4.
33. Lin HK, Lin SY, Li MJ, Compression force and amount of outer coating layer affecting the time controlled disintegration of the compression coated tablet prepared by direct compression with micronized ethylcellulose. J Pharm Sci 2001;90:2005-9.
Published
2014-08-03
Issue
Section
Original Article
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).

