Thyroid cytology evaluation based on the Bethesda system with clinico-morphological correlation
Keywords:
Thyroid, Cytology, TBSRTC, Histopathology, Accuracy
Abstract
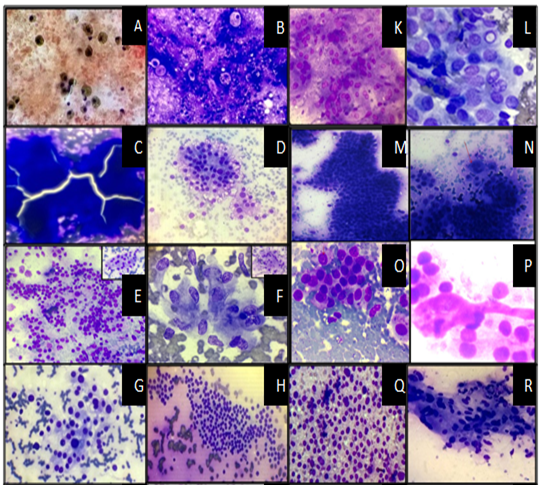
Background: FNAC of Thyroid gland is a widely accepted and accurate method for evaluation of thyroid nodules. In the past, terminology of reporting of Thyroid FNACs varied markedly, which made it difficult for the clinicians to interpret the reports and decide the management protocol. To cater to this issue, The Bethesda System of Reporting Thyroid Cytology was proposed with six diagnostic categories and appropriate management protocol for each category. This study was undertaken to categorize FNAC of Thyroid lesions according to the Bethesda System of Reporting Thyroid Cytology (TBSRTC) and to correlate with Histopathology wherever feasible.Methods: The present study includes 175 Thyroid FNAC cases studied over a 3-year period (August 2012- July 2015). These cases were categorized according to TBSRTC and the cytological diagnosis was correlated with histopathology wherever it was available. The Sensitivity, Specificity, Positive Predictive value (PPV), Negative Predictive value (NPV) and Accuracy was also calculated.Result: A total of 175 Thyroid FNAC cases were collected over 3 years. The mean age was 36.18 years and the male to female ratio was 1:9.3. Percentage of cases in Category I to VI according to the Bethesda system of reporting Thyroid cytology were 4.57%, 68.58%, 5.72%, 17.14%, 1.14% and 2.85% respectively. Histopathological details were available in 19.42% of the cases. The sensitivity, specificity, PPV, NPV and accuracy were 69.23%, 89.47%, 81.81%, 80.95% and 81.25% respectively.Conclusion: The findings of the present study were consistent with other studies that used TBSRTC.References
1. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol 2009; 132: 658-665.
2. Wang HH. Reporting thyroid fine-needle aspiration: Literature review and a proposal. Diagnostic Cytopathology 2006; 34(1): 67–76.
3. Parikh R, Mathai A, Parikh S, Chandra SG, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian Journal of Ophthalmology. 2008; 56(1):45-50.
4. Naz S, Hashmi AA, Khurshid A, Faridi N, Edhi MM, Kamal A, Khan M. Diagnostic accuracy of Bethesda system for reporting thyroid cytopathology: an institutional Perspective. International Archives of Medicine 2014; 7: 46-50.
5. Richmond BK, Judhan R, Chong B, Uber At, Aburahma Z, Mangano W, Stephanie Thompson. False-negative Results with the Bethesda System of Reporting Thyroid Cytopathology: Predictors of Malignancy in Thyroid Nodules Classified as Benign by Cytopathologic Evaluation. Am Surg. 2014; 80(8): 811–816.
6. Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007; 5: 306-315.
7. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007; 6: 508-516.
8. Joshi D, Jesalpura NS. Comparison between Bethesda System and Conventional System in Thyroid Cytopathology. IJSR. 2015; 4(9): 78-80.
9. Ko HM, Jhu IK, Yang SH, Lee JH, Nam JH, Juhng SW, et al. Clinicopathologic analysis of fine needle aspiration cytology of the thyroid. A review of 1,613 cases and correlation with histopathologic diagnoses. Acta Cytol. 2003; 47: 727–732.
10. Kessler A, Gavriel H, Zahav S, Vaiman M, Shlamkovitch N, Segal S, et al. Accuracy and consistency of fine-needle aspiration biopsy in the diagnosis and management of solitary thyroid nodules. Isr Med Assoc J. 2005; 7: 371–373.
11. Singh RS, Wang HH. Eliminating the “atypia of undetermined significance/follicular lesion of undetermined significance” category from the bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2011; 136:896–902.
12. Muratli A, Erdogan N, Sevim S, Unal I, Akyuz S. Diagnostic efficacy and importance of fine needle aspiration cytology of thyroid nodules. Journal of Cytology. 2014; 31:73-77.
13. Theoharis CG, Schofield KM, Hammers L, et al. The Bethesda thyroid fine-needle aspiration classification system:year 1 at an academic institution. Thyroid. 2009; 19: 1215-1223.
14. Nayar R, Ivanovic M. The indeterminate thyroid fine needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer Cytopathol. 2009; 11(7):195 -202.
15. Marchevsky AM, Walts AE, Bose S, et al. Evidence-based evaluation of the risks of malignancy predicted by thyroid fine needle aspiration biopsies. Diagn Cytopathol. 2010;38:252–259
16. Jo VY, Stelow EB, Dustin SM, Hanley KZ. Malignancy Risk for Fine Needle Aspiration of Thyroid Lesions According to The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2010; 134: 450- 456.
17. Renshaw AA. Should “atypical follicular cells” in thyroid fine-needle aspirates be subclassified? Cancer Cytopathol.2010; 118: 186-189.
18. VanderLaan PA, Marqusee E, Krane JF,. Clinical Outcome for Atypia of Undetermined Significance in Thyroid Fine-Needle Aspirations .Should Repeated FNA Be the Preferred Initial Approach?. Am J Clin Pathol 2011;135:770-775
19. Kim SK, Hwang TS, Yoo YB, et al. Surgical results of thyroid nodules according to a management guideline based on the BRAFV600E mutation status. J Clin Endocrinol Metab.2011; 96:658-664.
20. Krane JF, VanderLaan PA, Faquin WC, et al. The atypia of undetermined significance/follicular lesion of undetermined significance: malignant ratio: a proposed performance measure for reporting in The Bethesda System for Thyroid Cytopathology. Cancer Cytopathol. 2012; 120(2):111-116.
21. Mondal SK, Sinha S, Basak B, Roy DN, Sinha SK. The Bethesda system for reporting thyroid fine needle aspirates: A cytologic study with histologic follow up. Journal of Cytology.2013; 30(2): 94-99.
22. Mehra P, Verma AK. Thyroid Cytopathology Reporting by the Bethesda System:A Two-Year Prospective Study in an Academic Institution. Pathology Research International.2015, 1-11.
23. Mamatha M, Sekhar SC, et al.. A comparative study between conventional system and the Bethesda system applied for reporting thyroid cytopathology. International Archives of Integrated Medicine. 2015; 2(3): 87-95.
24. Melo-Uribe MA, Sanabria A, Romero-Rojas A, et al. The Bethesda system for reporting thyroid cytopathology in Colombia: Correlation with histopathological diagnoses in oncology and non-oncology institutions. Journal of Cytology. 2015; 32(1):12-16.
25. Al-Sayer HM, Krukowski ZH, Williams VM, Matheson NA. Fine needle aspiration cytology in isolated thyroid swellings: A prospective two year evaluation. Br Med J (Clin Res Ed) 1985; 290: 1490–1492.
26. Cusick EL, MacIntosh CA, Krukowski ZH, Williams VM, Ewen SW, Matheson NA. Management of isolated thyroid swellings: A prospective six year study of fine needle aspiration cytology in diagnosis. BMJ.1990; 301: 318–321.
27. Bouvet M, Feldman JI, Gill GN, Dillmann WH, Nahum AM, Russack V, et al. Surgical management of the thyroid nodule: Patient selection based on the results of fine-needle aspiration cytology. Laryngoscope.1992; 102:1353–1356.
28. Afroze N, Kayani N, Hasan SH. Role of fine needle aspiration cytology in the diagnosis of palpable thyroid lesions. Indian J Pathol Microbiol. 2002; 45: 241–246.
29. Al-Hureibi KA, Al-Hureibi AA, Abdulmughni YA, Aulaqi SM, Salman MS, Al-Zooba EM. The diagnostic value of fine needle aspiration cytology in thyroid swellings in a university hospital, Yemen. Saudi Med J. 2003; 24: 499–503.
30. Mahar SA, Husain A, Islam N. Fine needle aspiration cytology of thyroid nodule: Diagnostic accuracy and pitfalls. J Ayub Med Coll Abbottabad. 2006; 18: 26–29.
31. Haberal AN, Toru S, Ozen O, Arat Z, Bilezikçi B. Diagnostic pitfalls in the evaluation of fine needle aspiration cytology of the thyroid: Correlation with histopathology in 260 cases. Cytopathology.2009; 20: 103–108.
2. Wang HH. Reporting thyroid fine-needle aspiration: Literature review and a proposal. Diagnostic Cytopathology 2006; 34(1): 67–76.
3. Parikh R, Mathai A, Parikh S, Chandra SG, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian Journal of Ophthalmology. 2008; 56(1):45-50.
4. Naz S, Hashmi AA, Khurshid A, Faridi N, Edhi MM, Kamal A, Khan M. Diagnostic accuracy of Bethesda system for reporting thyroid cytopathology: an institutional Perspective. International Archives of Medicine 2014; 7: 46-50.
5. Richmond BK, Judhan R, Chong B, Uber At, Aburahma Z, Mangano W, Stephanie Thompson. False-negative Results with the Bethesda System of Reporting Thyroid Cytopathology: Predictors of Malignancy in Thyroid Nodules Classified as Benign by Cytopathologic Evaluation. Am Surg. 2014; 80(8): 811–816.
6. Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007; 5: 306-315.
7. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007; 6: 508-516.
8. Joshi D, Jesalpura NS. Comparison between Bethesda System and Conventional System in Thyroid Cytopathology. IJSR. 2015; 4(9): 78-80.
9. Ko HM, Jhu IK, Yang SH, Lee JH, Nam JH, Juhng SW, et al. Clinicopathologic analysis of fine needle aspiration cytology of the thyroid. A review of 1,613 cases and correlation with histopathologic diagnoses. Acta Cytol. 2003; 47: 727–732.
10. Kessler A, Gavriel H, Zahav S, Vaiman M, Shlamkovitch N, Segal S, et al. Accuracy and consistency of fine-needle aspiration biopsy in the diagnosis and management of solitary thyroid nodules. Isr Med Assoc J. 2005; 7: 371–373.
11. Singh RS, Wang HH. Eliminating the “atypia of undetermined significance/follicular lesion of undetermined significance” category from the bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2011; 136:896–902.
12. Muratli A, Erdogan N, Sevim S, Unal I, Akyuz S. Diagnostic efficacy and importance of fine needle aspiration cytology of thyroid nodules. Journal of Cytology. 2014; 31:73-77.
13. Theoharis CG, Schofield KM, Hammers L, et al. The Bethesda thyroid fine-needle aspiration classification system:year 1 at an academic institution. Thyroid. 2009; 19: 1215-1223.
14. Nayar R, Ivanovic M. The indeterminate thyroid fine needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer Cytopathol. 2009; 11(7):195 -202.
15. Marchevsky AM, Walts AE, Bose S, et al. Evidence-based evaluation of the risks of malignancy predicted by thyroid fine needle aspiration biopsies. Diagn Cytopathol. 2010;38:252–259
16. Jo VY, Stelow EB, Dustin SM, Hanley KZ. Malignancy Risk for Fine Needle Aspiration of Thyroid Lesions According to The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2010; 134: 450- 456.
17. Renshaw AA. Should “atypical follicular cells” in thyroid fine-needle aspirates be subclassified? Cancer Cytopathol.2010; 118: 186-189.
18. VanderLaan PA, Marqusee E, Krane JF,. Clinical Outcome for Atypia of Undetermined Significance in Thyroid Fine-Needle Aspirations .Should Repeated FNA Be the Preferred Initial Approach?. Am J Clin Pathol 2011;135:770-775
19. Kim SK, Hwang TS, Yoo YB, et al. Surgical results of thyroid nodules according to a management guideline based on the BRAFV600E mutation status. J Clin Endocrinol Metab.2011; 96:658-664.
20. Krane JF, VanderLaan PA, Faquin WC, et al. The atypia of undetermined significance/follicular lesion of undetermined significance: malignant ratio: a proposed performance measure for reporting in The Bethesda System for Thyroid Cytopathology. Cancer Cytopathol. 2012; 120(2):111-116.
21. Mondal SK, Sinha S, Basak B, Roy DN, Sinha SK. The Bethesda system for reporting thyroid fine needle aspirates: A cytologic study with histologic follow up. Journal of Cytology.2013; 30(2): 94-99.
22. Mehra P, Verma AK. Thyroid Cytopathology Reporting by the Bethesda System:A Two-Year Prospective Study in an Academic Institution. Pathology Research International.2015, 1-11.
23. Mamatha M, Sekhar SC, et al.. A comparative study between conventional system and the Bethesda system applied for reporting thyroid cytopathology. International Archives of Integrated Medicine. 2015; 2(3): 87-95.
24. Melo-Uribe MA, Sanabria A, Romero-Rojas A, et al. The Bethesda system for reporting thyroid cytopathology in Colombia: Correlation with histopathological diagnoses in oncology and non-oncology institutions. Journal of Cytology. 2015; 32(1):12-16.
25. Al-Sayer HM, Krukowski ZH, Williams VM, Matheson NA. Fine needle aspiration cytology in isolated thyroid swellings: A prospective two year evaluation. Br Med J (Clin Res Ed) 1985; 290: 1490–1492.
26. Cusick EL, MacIntosh CA, Krukowski ZH, Williams VM, Ewen SW, Matheson NA. Management of isolated thyroid swellings: A prospective six year study of fine needle aspiration cytology in diagnosis. BMJ.1990; 301: 318–321.
27. Bouvet M, Feldman JI, Gill GN, Dillmann WH, Nahum AM, Russack V, et al. Surgical management of the thyroid nodule: Patient selection based on the results of fine-needle aspiration cytology. Laryngoscope.1992; 102:1353–1356.
28. Afroze N, Kayani N, Hasan SH. Role of fine needle aspiration cytology in the diagnosis of palpable thyroid lesions. Indian J Pathol Microbiol. 2002; 45: 241–246.
29. Al-Hureibi KA, Al-Hureibi AA, Abdulmughni YA, Aulaqi SM, Salman MS, Al-Zooba EM. The diagnostic value of fine needle aspiration cytology in thyroid swellings in a university hospital, Yemen. Saudi Med J. 2003; 24: 499–503.
30. Mahar SA, Husain A, Islam N. Fine needle aspiration cytology of thyroid nodule: Diagnostic accuracy and pitfalls. J Ayub Med Coll Abbottabad. 2006; 18: 26–29.
31. Haberal AN, Toru S, Ozen O, Arat Z, Bilezikçi B. Diagnostic pitfalls in the evaluation of fine needle aspiration cytology of the thyroid: Correlation with histopathology in 260 cases. Cytopathology.2009; 20: 103–108.
Published
2016-10-26
Issue
Section
Original Article
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access at http://opcit.eprints.org/oacitation-biblio.html).




