Assessemnt of Biochemical, Serological, Molecular Viral Marker and Histological Parameters in HBeAg Positive Chronic Hepatitis B to Determine Therapy Response
Keywords:
HBeAg, chronic hepatitis B, therapy, serological and viral markers, histology
Abstract
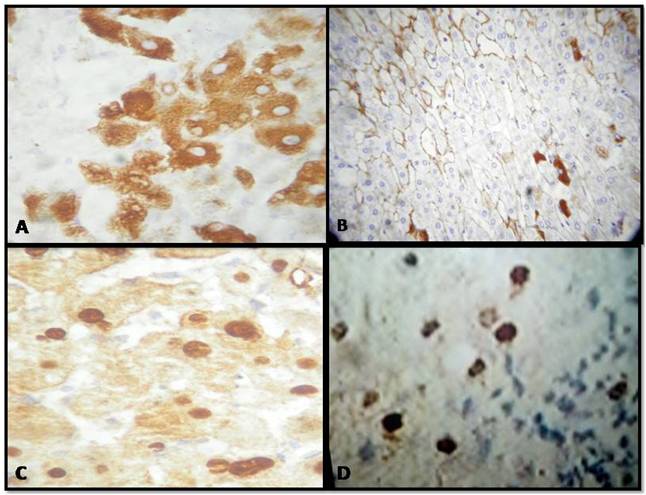
Introduction: About 300 million people worldwide have chronic hepatitis B virus (HBV) infection with varying degree of liver damage. The presence of continuing viral replication correlates with continuing disease activity and is associated with Hepatitis B e antigen (HBeAg) and hepatitis B virus DNA (HBV-DNA) in serum. Subsequently, the patient may undergo a spontaneous or therapy induced remission, which is accompanied by loss of HBV-DNA and HBeAg. This prospective study was undertaken to correlate all the above parameters so as to have an insight to monitoring of therapy in HBeAg positive chronic hepatitis B (CHB).Aims and objectives: 1. To determine changes in biochemical, serological and virological profile in HBeAg positive CHB with therapy. 2. Determination of histology in liver biopsies in all cases and correlation with immunohistochemical detection of HBsAg and HBcAg with above parameters.Methods: 42 patients of HBeAg positive CHB were enrolled and were followed up for 24 months. Blood samples were collected for alanine aminotransferase (ALT), hepatitis B surface antigen (HBsAg), Anti-hepatitis B core antigen (HBcAg), HBeAg and HBV-DNA. Liver biopsies were done in all individuals. Immunohistochemical staining for HBsAg and HBcAg were done where indicated. The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 statistical Analysis Software.Results: There was a statistically significant improvement in the biochemical, serological and virological profile of the patients after therapy. However, the necroinflammatory activity showed improvement but was not statistically significant. Immunohistochemistry showed good correlation with viral load.References
1. Lee WM. Hepatitis B virus infection. New Eng J Med 1997;24:1733-1745.
2. World Health Organization: WHO: Hepatitis B. Fact Sheet No.204. (http://www.who.int/mediacentre/factsheets/fs204/en/).
3. Lok AS: Chronic hepatitis B. N Engl J Med. 2002;346(22):1682-3.
4. Koyuncuer A. Associations between HBeAg Status, HBV DNA, ALT Level and Liver Histopathology in Patients with Chronic Hepatitis B. Science Journal of Clinical Medicine. 2014;3:117-123.
5. Sharma SK, Saini N, Chawla Y: Hepatitis B virus: inactive carriers. Virol J. 2005; 2:82.
6. Squadrito G, Spinella R, Raimondo G: The clinical significance of occult HBV infection. Ann Gastroenterol. 2014;27(1):15-19.
7. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006; 43(2 Suppl 1):S173-81.
8. Geller SA, Petrovic LM. Chronic Hepatitis (Chronic Necroinflammatory Disease of the Liver)-Grading and Staging. Biopsy Interpretation of the Liver. 2nd edition. Philadelphia: Lippincott Williams & Wilkins; 2009;97-120.
9. Badur S, Akgun A. Diagnosis of hepatitis B infections and monitoring of treatment. J Clin Virol. 2001; 21: 229-37.
10. Dienstag JL: Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology 2009, 49:S112–S121.
11. Andersson KL, Chung RT. Monitoring During and After Antiviral Therapy for Hepatitis B. Hepatology. 2009 ; 49: 166–173.
12. Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007; 45:1056–1075.
13. Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001; 120:1828–1853.
14. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995 Jun;22(6):696-699.
15. Hussain AB, Karamat KA, Anwar M, Kazmi SY, Tariq WU. Correlation of HBV DNA PCR and HBeAg in hepatitis carriers. J Coll Physicians Surg Pak. 2004; 14: 18-20.
16. Pincus MR, Tierno PM, Fenelus M, Bowne WB, Bluth MH. Evaluation of liver function and injury. In Henry JB: Clinical diagnosis and management by laboratory methods, El siever. 22th edition; Chapter 21: 296-311.
17. Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998; 339:61–68.
18. Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008; 134:1376–1384.
19. Zeng Y, Yang B, Wu Y, Chen J, Shang H, Chen X et al. Clinical significance of periodic detection of hepatitis B virus YMDD mutation by ultrasensitive real-time amplification refractory mutation system quantitative PCR during lamivudine treatment in patients with chronic hepatitis B. Journal of Medical Microbiology (2015), 64, 237–242.
20. Feld JJ, Wong DK, Heathcote EJ: Endpoints of therapy in chronic hepatitis B. Hepatology 2009, 49:S96–S102.
21. Yang J, Chen J, Ye P, Jin L, Wu W, Sheng G, Li L. HBsAg as an important predictor of HBeAg seroconversion following antiviral treatment for HBeAg-positive chronic hepatitis B patients. Journal of Translational Medicine 2014, 12:183-191.
22. Ryu SH, Chung YH, Choi MH, Kim JA, Shin JW, Jang MK, Park NH, et al. Long-term additional lamivudine therapy enhances durability of lamivudine-induced HBeAg loss: a prospective study. J Hepatol. 2003; 39:614–619.
23. Hou FQ, Song LW, YuanQ, Fang LL, Ge SX, Zhang J et al. Quantitative Hepatitis B Core Antibody Level Is a New Predictor for Treatment Response In HBeAg-positive
Chronic Hepatitis B Patients Receiving Peg-interferon. Theranostics 2015;5:218-226.
24. Colloredo G, Bellati G, Sonzogni A, Zavaglia C, Fracassetti O, Leandro G et al. Semiquantitative assessment of IgM antibody to hepatitis B core antigen and prediction of severity of chronic hepatitis B. J viral Hepatitis 1999;6:429-434.
25. Harrison TJ, Anderson MG, Murray-Lyon IM, Zuckerman AJ. Hepatitis B virus in hepatocytes: a series of 160 biopsies. J Hepatol 1986;2:1-10.
26. Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007; 357:2576–2588.
27. Yuan HJ, Yuen MF, Ka-Ho Wong D, Sum SM, Sablon E, Oi-Lin Ng I, Lai CL. Impact of precore and core promoter mutations on hepatic histology in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2005;22:301-307.
28. Omata M, Yokosuka O, Imazeki F, Ito Y, Mori J, Uchiumi K et al. Correlation of hepatitis B virus DNA and antigens in the liver. Gastroenetrology 1987;92:192-196.
29. Ramalho F, Brunetto MR, Rocca G, Piccari GG, Batista A, Chiaberge E et al. Serum markers of hepatitis B virus replication, liver histology and intrahepatic expression of hepatitis B core antigen. J Hepatol 1988;7:14-20.
30. Lindh M, Savage K, Rees J, Garwood L, Horal P, Norkrans G et al. HBeAg immunostaining of liver tissue in various stages of chronic hepatitis B. Liver 1999;19:294-298.
2. World Health Organization: WHO: Hepatitis B. Fact Sheet No.204. (http://www.who.int/mediacentre/factsheets/fs204/en/).
3. Lok AS: Chronic hepatitis B. N Engl J Med. 2002;346(22):1682-3.
4. Koyuncuer A. Associations between HBeAg Status, HBV DNA, ALT Level and Liver Histopathology in Patients with Chronic Hepatitis B. Science Journal of Clinical Medicine. 2014;3:117-123.
5. Sharma SK, Saini N, Chawla Y: Hepatitis B virus: inactive carriers. Virol J. 2005; 2:82.
6. Squadrito G, Spinella R, Raimondo G: The clinical significance of occult HBV infection. Ann Gastroenterol. 2014;27(1):15-19.
7. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006; 43(2 Suppl 1):S173-81.
8. Geller SA, Petrovic LM. Chronic Hepatitis (Chronic Necroinflammatory Disease of the Liver)-Grading and Staging. Biopsy Interpretation of the Liver. 2nd edition. Philadelphia: Lippincott Williams & Wilkins; 2009;97-120.
9. Badur S, Akgun A. Diagnosis of hepatitis B infections and monitoring of treatment. J Clin Virol. 2001; 21: 229-37.
10. Dienstag JL: Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology 2009, 49:S112–S121.
11. Andersson KL, Chung RT. Monitoring During and After Antiviral Therapy for Hepatitis B. Hepatology. 2009 ; 49: 166–173.
12. Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007; 45:1056–1075.
13. Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001; 120:1828–1853.
14. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995 Jun;22(6):696-699.
15. Hussain AB, Karamat KA, Anwar M, Kazmi SY, Tariq WU. Correlation of HBV DNA PCR and HBeAg in hepatitis carriers. J Coll Physicians Surg Pak. 2004; 14: 18-20.
16. Pincus MR, Tierno PM, Fenelus M, Bowne WB, Bluth MH. Evaluation of liver function and injury. In Henry JB: Clinical diagnosis and management by laboratory methods, El siever. 22th edition; Chapter 21: 296-311.
17. Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998; 339:61–68.
18. Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008; 134:1376–1384.
19. Zeng Y, Yang B, Wu Y, Chen J, Shang H, Chen X et al. Clinical significance of periodic detection of hepatitis B virus YMDD mutation by ultrasensitive real-time amplification refractory mutation system quantitative PCR during lamivudine treatment in patients with chronic hepatitis B. Journal of Medical Microbiology (2015), 64, 237–242.
20. Feld JJ, Wong DK, Heathcote EJ: Endpoints of therapy in chronic hepatitis B. Hepatology 2009, 49:S96–S102.
21. Yang J, Chen J, Ye P, Jin L, Wu W, Sheng G, Li L. HBsAg as an important predictor of HBeAg seroconversion following antiviral treatment for HBeAg-positive chronic hepatitis B patients. Journal of Translational Medicine 2014, 12:183-191.
22. Ryu SH, Chung YH, Choi MH, Kim JA, Shin JW, Jang MK, Park NH, et al. Long-term additional lamivudine therapy enhances durability of lamivudine-induced HBeAg loss: a prospective study. J Hepatol. 2003; 39:614–619.
23. Hou FQ, Song LW, YuanQ, Fang LL, Ge SX, Zhang J et al. Quantitative Hepatitis B Core Antibody Level Is a New Predictor for Treatment Response In HBeAg-positive
Chronic Hepatitis B Patients Receiving Peg-interferon. Theranostics 2015;5:218-226.
24. Colloredo G, Bellati G, Sonzogni A, Zavaglia C, Fracassetti O, Leandro G et al. Semiquantitative assessment of IgM antibody to hepatitis B core antigen and prediction of severity of chronic hepatitis B. J viral Hepatitis 1999;6:429-434.
25. Harrison TJ, Anderson MG, Murray-Lyon IM, Zuckerman AJ. Hepatitis B virus in hepatocytes: a series of 160 biopsies. J Hepatol 1986;2:1-10.
26. Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007; 357:2576–2588.
27. Yuan HJ, Yuen MF, Ka-Ho Wong D, Sum SM, Sablon E, Oi-Lin Ng I, Lai CL. Impact of precore and core promoter mutations on hepatic histology in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2005;22:301-307.
28. Omata M, Yokosuka O, Imazeki F, Ito Y, Mori J, Uchiumi K et al. Correlation of hepatitis B virus DNA and antigens in the liver. Gastroenetrology 1987;92:192-196.
29. Ramalho F, Brunetto MR, Rocca G, Piccari GG, Batista A, Chiaberge E et al. Serum markers of hepatitis B virus replication, liver histology and intrahepatic expression of hepatitis B core antigen. J Hepatol 1988;7:14-20.
30. Lindh M, Savage K, Rees J, Garwood L, Horal P, Norkrans G et al. HBeAg immunostaining of liver tissue in various stages of chronic hepatitis B. Liver 1999;19:294-298.
Published
2016-11-06
Section
Original Article
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access at http://opcit.eprints.org/oacitation-biblio.html).




